 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeA gas cylinder containing cooking gas can withstand a pressure of 14.9 bar. The pressure gauge of the cylinder indicates 12 bar at 27°C. Due to sudden fire in the building, the temperature starts rising. At what temperature will the cylinder explode?
State Avogadro's law and prove that volume of gases at constant temperature and pressure is directly proportional to their number of moles.
 Short Answer Type
Short Answer TypeTwo flasks A and B have equal to volumes. Flask A contains H2 and is maintained at 300K while the flask B contains an equal mass of CH4 gas and is maintained at 600K. Which flask contains a greater number of molecules ? How many times?
 Long Answer Type
Long Answer TypeWith the help of Boyle's and Charle's laws, derive an expression for the ideal gas equation.
Derivation of the ideal gas equation: Let the volume of a given mass of a gas change from V1 to V2 when the pressure is changed from Pi to P2 and temperature is changed from T1 to T2. Suppose this change from initial state (P1 V1T1) to final state (P2V2T2) occurs in two steps:
First step: Suppose the volume of the given mass of the gas changes from V1 to Vx when pressure is changed from P1 to P2, keeping temperature T1 constant.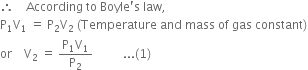
Second step: Now let the volume changed from Vx to V2 when the temperature is changed from T1 to T2, keeping pressure P2 constant.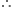 According to Charle's law,
According to Charle's law,
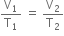
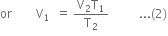
From (1) and (2), we have

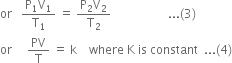
The numerical value of the constant K is independent of the nature of the gas but depends on upon the amount of the gas. But at constant temperature and pressure the volume of a gas is proportional to the number of moles (n), this means that K is directly proportional to the number of moles (n).
where R is a constant of proportionality which is independent of nature as well as the amount of the gas and is known as a universal gas constant. From (4) and (5)
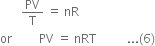
Equation (6) is known as an ideal gas equation. Alternative derivation of ideal gas equation
: According to Boyle’s law,

According to Charle's law,

According to Avogadro's law,

Combining (1), (2) and (3), we have,

.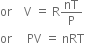
 Short Answer Type
Short Answer TypeDerive the dimensions of the gas constant.
Or
What do you mean by the nature of universal consant R?
How much time would it take to distribute one Avogadro number of wheat grains, if 1010grains are distributed each second?
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeUsing the equation of state pV = nRT; show that at a given temperature density of a gas is proportional to gas pressure p.
