 Long Answer Type
Long Answer Type250 mL of a gas is confined in a vessel at at 15°C and 750 mm pressure. How much volume will it occupy at S.T.P.?
A sample of nitrogen gas occupies a volume of 1.00L at a pressure of 0.50 bar and at 40°C. Calculate the pressure if the gas is compressed to 0·225 cm3 at –6°C.
At 25°C and 760 mm of Hg pressure, a gas occupies 600 mL volume. What will be its pressure at a height where the temperature is 10°C and volume of the gas are 640 mL ?
To what temperature must a neon gas sample be heated to double its pressure, if the initial volume of the gas at 75°C is decreased by 15%?
 Short Answer Type
Short Answer TypeThe drain cleaner, Drainex contains small bits of aluminium which react with caustic soda to produce dihydrogen. What volume of dihydrogen at 20°C and one bar will be released when 0·15g of aluminium reacts?
Al reacts with caustic soda to produce dihydrogen.
54g Al on reacting with caustic soda produces
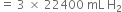
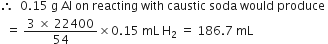
Applying the gas equation
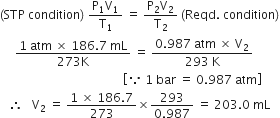
Calculate the volume occupied by 7.0 g of nitrogen gas at 27°C and 750 mm Hg pressure.
Calculate the volume occupied by 8.8 g of CO2 at 31·1°C and 1 bar pressure. R = 0.083 bar LK–1 mol–1).
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type