 Long Answer Type
Long Answer Type250 mL of a gas is confined in a vessel at at 15°C and 750 mm pressure. How much volume will it occupy at S.T.P.?
A sample of nitrogen gas occupies a volume of 1.00L at a pressure of 0.50 bar and at 40°C. Calculate the pressure if the gas is compressed to 0·225 cm3 at –6°C.
At 25°C and 760 mm of Hg pressure, a gas occupies 600 mL volume. What will be its pressure at a height where the temperature is 10°C and volume of the gas are 640 mL ?
To what temperature must a neon gas sample be heated to double its pressure, if the initial volume of the gas at 75°C is decreased by 15%?
 Short Answer Type
Short Answer TypeThe drain cleaner, Drainex contains small bits of aluminium which react with caustic soda to produce dihydrogen. What volume of dihydrogen at 20°C and one bar will be released when 0·15g of aluminium reacts?
Calculate the volume occupied by 7.0 g of nitrogen gas at 27°C and 750 mm Hg pressure.
Calculate the volume occupied by 8.8 g of CO2 at 31·1°C and 1 bar pressure. R = 0.083 bar LK–1 mol–1).
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeWe know,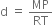
At the same temperature and for same density

(Gaseous oxide) (N2)
Here P1 = 2 bar;
P2 = 5 bar;
M2 = molecular mass of N2 = 28
Substituting the values in equation(1), we have
M1 x 2 = 28 x 5
