 Long Answer Type
Long Answer TypeWhat is the effect of temperature on the distribution of molecular velocities?
On increasing the temperature, the motion of the gas molecules becomes rapid and hence the value of most probable velocity also increases. As a result, the entire distribution curve becomes flatter and peak shifts to regions of higher velocities as shown in the figure.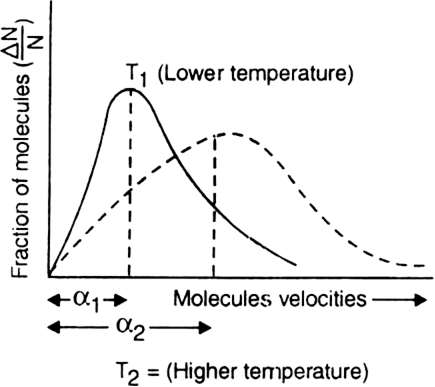
α1 = Most probable velocity at temp. T1
α2 = Most probable velocity at temp. T2
α2 > α1
 Short Answer Type
Short Answer TypeEven though carbon dioxide is heavier than air, it does not form the lower layer, Explain.
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeWhat are the faulty assumptions in the kinetic theory of gases which are responsible for deviations from ideal behaviour of gases?
What modifications were applied by Vander Waal to overcome the deviations of the gases from ideal gas behaviour?
 Long Answer Type
Long Answer TypeWhat is the significance of the Vander Waal's constants? Write its units for Vander Waal's constants.
