 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeEven though carbon dioxide is heavier than air, it does not form the lower layer, Explain.
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeWhat are the faulty assumptions in the kinetic theory of gases which are responsible for deviations from ideal behaviour of gases?
What modifications were applied by Vander Waal to overcome the deviations of the gases from ideal gas behaviour?
 Long Answer Type
Long Answer TypeWhat is the significance of the Vander Waal's constants? Write its units for Vander Waal's constants.
Vander Waal's equation of state for n moles of the gas is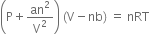
The significance of constant 'a'. The value of constant a gives the idea of the magnitude of attractive forces between the molecules of the gas. Larger the value of a the larger will be the intermolecular forces between the molecules. Units of a. Pressure correction (β) is given by
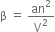
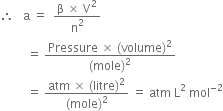
The significance of constant 'b'. The constant b is called co-volume or excluded volume per mole of a gas. It is a measure of the effective size of gas molecules.
Units of b. Units of b must be units of volume i.e. L mol–1 or dm3 mol–1.
