 Short Answer Type
Short Answer TypeExplain the following:
(i) Drops of liquid assume a spherical shape.
(ii) The level of mercury in a capillary tube is lower than the level outside when a capillary tube is inserted in mercury.
Liquid that wets glass rises in a capillary tube or oil rises in the wick of an oil Jamp. Explain.
 Long Answer Type
Long Answer TypeExplain the following:
(i) The boiling point of water (373 K) is abnormally high when compared to that of H2S (211·2K).
(ii) Liquids like ether and acetone are kept in cool places.
(iii) Tea or coffee is sipped from a saucer when it is quite hot.
Explain briefly the term viscosity. Define coefficient of viscosity. What are its units?
 Short Answer Type
Short Answer TypeWhich one in each of the following pairs is more viscous?
(i) Coconut oil, castor oil
(ii) glyercine, kerosene
(iii) soft drink, aerated water (soda water)?
 Long Answer Type
Long Answer TypeWhat is the effect of temperature on:
(i) density
(ii) surface tension
(iii) viscosity
(iv) the vapour pressure of a liquid?
(i) Density: The density of a liquid generally decreases with the increase in temperature because liquids expand on heating i.e. volume increases and therefore, 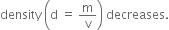
(ii) Surface tension. The surface tension of a liquid decreases with the increase in temperature because with the rise in temperature, the kinetic energy of the molecules increases and therefore, intermolecular attraction decreases.
(iii) Viscosity: The viscosity of a liquid decreases with the increase in temperature because with the rise in temperature, the average kinetic energy of the molecules increases which can overcome the intermolecular forces.
(iv) Vapour pressure: Vapour pressure of a liquid increases with the increase in temperature because with the rise in the temperature, the average kinetic energy of the liquid molecules increases. Thus, the number of energetic molecules capable of escaping into the vapour state also increases. As a result, the number of molecules present in the vapour state at equilibrium will also be more. Consequently, the vapour pressure will naturally be greater.
 Short Answer Type
Short Answer TypeWhat is the effect of pressure on:
(i) volume
(ii) boiling point
(iii) viscosity of a liquid?
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type