 Multiple Choice Questions
Multiple Choice QuestionsA 0.66 kg ball is moving with a speed of 100 m/s. The associated wavelength will be
(h = 6.6 x 10-34 Js)
6.6 x 10-32 m
6.6 x 10-34 m
1 x 10-35 m
1 x 10-35 m
Maximum number of electrons in a subshell of an atom is determined by the following
4l + 2
2l +1
4l-2
4l-2
The energy absorbed by each molecule (A2) of a substance is 4.4 x 10-19 J and bond energy per molecule is 4.0 X 10-19 J. The kinetic energy of the molecule per atom will be
2.0 x 10-20 J
2.2 x 10-19 J
2.0 x 10-19 J
2.0 x 10-19 J
Which of the following is not permissible arrangement of electrons in an atom?
n = 4, l=0, m=0, s=-1/2
n=5, l=3, m=0, s=+1/2
n=3, l=2, m=-3, s= -1/2
n=3, l=2, m=-3, s= -1/2
The measurment of the electron position is associated with an uncerrtanity in momentum, which equal to 1 x 10-18 g cm s-1.The uncertanity in electron velocity is,
(mass of an electrons is 9 x 10-28 g)
1 x 108
1 x 106 cm s-1
1 x 105 cm s-1
1 x 105 cm s-1
Given: The mass of electron is 9.11 x 10-31 kg
Planck constant is 6.626 x 10-34 Js,
the uncertainty involved in the measurement of velocity within a distance of 0.1 A is:
5.79 x 106 ms-1
5.79 x 107 ms-1
5.79 x 108 ms-1
5.79 x 108 ms-1
The orientation of an atomic orbital is governed by:
azimuthal quantum number
spin quantum number
magnetic quantum number
magnetic quantum number
Which one is the wrong statement?
de-Broglie's wavelength is given by λ = h/mv,
where m = mass of the particle, v = group velocity of the particle
The uncertainty principle is 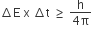
Half-filled and fully filled orbitals have greater stability due to greater exchange energy, greater symmetry and more balanced arrangement
Half-filled and fully filled orbitals have greater stability due to greater exchange energy, greater symmetry and more balanced arrangement
Which one is a wrong statement?
Total orbital angular momentum of electron in 's' orbital is equal to zero
An orbital is designated by three quantum numbers while an electron in an atom is designated by four quantum numbers
The value of m for dz2 is zero
The electronic configuration of N atom is

