 Short Answer Type
Short Answer TypeGive a mathematical relation between wavelength and momentum of a particle.
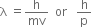
The wavelength of a proton, an electron and an α-particle moving with the same velocity are in the order: electron > proton > α-particle. How do you justify it?
