 Short Answer Type
Short Answer TypeGive the number of electrons in the species ![]()
No. of electrons in 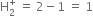
Number of electrons in 
Number of electrons in 
Write the complete symbol for the atom with the given atomic number (Z) and atomic number (A).
(i) Z = 17, A = 35
(ii) Z = 92, A = 233
(iii) Z = 4, A = 9.
What is the atomic number and mass number of A3+ ion with 21 electrons and 25 neutrons?
Isotopes of an element have similar chemical properties and isobars have different chemical properties. Explain.
