 Short Answer Type
Short Answer TypeIf the diameter of a carbon atom is 0.15 nm, calculate the number of carbon atoms which can be placed side by side in a straight line across length of scale of length 20 cm long.
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeA certain particle carries 2.5 X 10-16C of static electric charge. Calculate the number of electrons present in it.
In Milikan's experiment, static electric charge on the oil drops has been obtained by shining X-rays. If the static electric charge on the oil drop is - 1.282 X 10-18C, calculate the number of electrons present on it.
 Long Answer Type
Long Answer TypeFind: (a) the total number and (b) the total mass of protons in 34 mg of NH3 at STP.
Will the answer change if the temperature and pressure are changed ?
(a) 1 molecule of NH3 contains
= 7 + 3 = 10 protons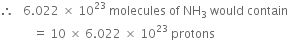
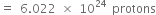
Now 17g NH3 contains 6.022 x 1024 protons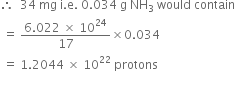
(b) Mass of one proton = 1.6726 x 10-27 kg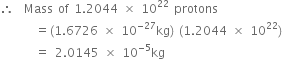
There is no effect of temperature and pressure.
