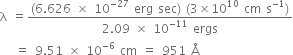Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeHow does Bohr's model of atom explain the atomic spectra of hydrogen and hydrogenlike particles?
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWhat are frequency and wavelength of a photon emitted during a transition from n=5 state to n = 2 state in hydrogen atom?
What is the wavelength of ligh emitted when the election in a hydrogen atom undergoes transition from an energy level with n = 4 to an energy level with n = 2?
What is the energy in joules, required to shift the electron of the hydrogen atom from the first Bohr orbit to the fifth Bohr orbit and what is the wavelength of the light emitted when the electron returns to the ground state? The ground state electron energy is - 2.18 X 10-11 ergs
As ground state, electronic energy is 
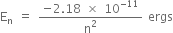
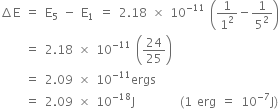
When electron returns to ground state (i.e. to n = 1), energy emitted 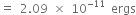
We know,

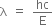
Substituting the values in the above expression