 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeHow will you correlate Bohr’s concept of hydrogen atom with de-Broglie concept?
Or
Show that the circumference of Bohr orbit for the hydrogen atom is an integral multiple of de-Broglie wavelength associated with electron revolving around the orbit.
What will be the wavelength of a ball of mass 0.1 kg moving with a velocity of 10 ms-1?
According to de-Broglie equation

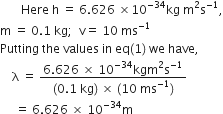
If the velocity of the electron in Bohr's first orbit is 2.19 x 106 ms-1, calculate the de Broglie wavelength associated with it.
The velocity associated with a proton moving in a potential difference of 1000V is 4.37 x 105 ms-1. If the hockey ball of mass 0.1 kg is moving with this velocity, calculate the wavelength associated with this velocity.
Dual behaviour of matter proposed by de Broglie led to the discovery of electron microscope often used for the highly magnified images of biological molecules and other type of material. If the velocity of the leectron in this microscope is 1.6 X 106 ms-1, calculate de Broglie wavelength associated with this electron.
