 Short Answer Type
Short Answer TypeUsing s, p, d notations describe the orbital with the following quantum numbers :
(a) n = 1, l = 0,
(b) n = 3, l = 1,
(c) n = 4, l = 2,
(d) n = 4, l = 3.
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
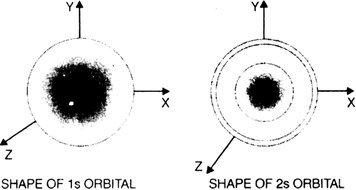
Long Answer TypeDiscuss the shapes of s-orbitals.
 Short Answer Type
Short Answer TypeThe bromine atom possesses 35 electrons. It contains 6 electrons in 2p orbital, 6 electrons in 3p orbital and 5 electrons in 4p orbital. Which of these electron experiences the lowest effective nuclear charge?
