 Long Answer Type
Long Answer TypeWrite down the electronic configuration of elements from atomic number 21 to atomic number 30. Also name the elements.
How will you account for the electronic configuration of the elements with atomic number of 24 and 29?
Or
Account for the following:
The continuous building up of 3d subshell interpreted in chromium (At. No. 24) and copper (At No. 29).
Or
Outer electronic configuration of chromium is 3d5 4s1 and not 3d4 4s2. Explain.
Atomic number (24):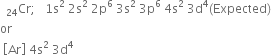
The above configuration is less symmetrical and also less stable because four 3d orbitals are half killed while one is empty. But the actual electronic configuration of chromium is: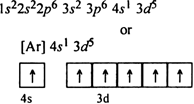
Since all the five 3d orbitals are half filled so this configuration is more symmetrical and also more stable.
(ii) Atomic number (29)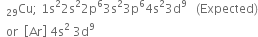
But the actual electronic configuration of copper is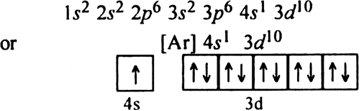
i.e. all the 3d orbitals get completely filled and 4s orbital is exactly half filled. Hence this configuration is more symmetrical and also more stable.
 Short Answer Type
Short Answer TypeWrite the electronic configurations of the following ions: (a)H- (b) Na+ (c) O2- (d) F-
Which atoms are indicated by the following configurations?
(a) [He] 2s1
(b) [Ne] 3s2 3p3
(c) [Ar] 4s2 3d1.
Which of the following are isoelectronic species, i.e. those having the same number of electrons?
Na+, K+ , Mg2+, Ca2+, S2-, Ar.
What is the cause of greater stability of exactly half-filled and completely filled configurations?
An atom of an element contains 29 electrons and 35 Deutrons. Deduce (i) the number of protons and (ii) the electronic configuration of the element.
 Long Answer Type
Long Answer TypeWrite the electronic configurations of the following ions/atom:
(i) Cu2+ (ii) Cr3+
(iii) Ni2+ (iv) Rubidium (Z = 37)
