 Multiple Choice Questions
Multiple Choice QuestionsThe work functions of Ag, Mg, K and Na respectively in eV are 4.3, 3.7, 2.25, 2.30. When an electromagnetic radiation of wavelength of 300 nm is allowed to fall on these metal surface, the number of metals from which the electrons are ejected is (1eV = 1.6022 × 10-19 J).
4
3
2
5
The values for all the quantum numbers for 15th electron of chlorine are
n = 3; l = 1; m = 0; s = -
n = 4; l = 2; m = 0; s = +
n = 3; l = 1; m = +1; s = +
n = 2; l = 0; m = 0; s = +
C.
n = 3; l = 1; m = +1; s = +
Electronic configuration of chlorine is 17Cl - 1s2, 2s2 2p6, 3s2 3p5.
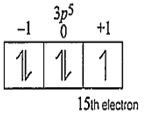
n = 3; l = 1; m = +1 and s = +
Which of the following is incorrect for radial distribution curve?
n = 2; l = 0; Node = 1
n = 3; l = 0; Node = 2
n = 2; l = 1; Node = 0
n = 3; l = 2; Node = 1
Number of α and β emitted in the reaction, 92U238 → 82Pb206 are
6α and 8β
8α and 6β
6α and 4β
4α and 6β
Which of the following describes the shape of orbital?
Principal quantum number
Azimuthal quantum number
Magnetic quantum number
Spin quantum number
For an electron impossible combination of quantum number is
n = 3; l = 2; ml = -2; ms = +
n = 3; l = 2; ml = -3; ms = +
n = 4; l = 0; ml = 0; ms = -
n = 5; l = 3; ml = 0; ms = -
