 Multiple Choice Questions
Multiple Choice QuestionsThe set of quantum numbers for the outermost electron for copper in its ground state is
4, 1, 1 +1/2
3, 2, 2, +1/2
4, 0, 0, +1/2
4, 2, 2, +1/2
Which one of the following sets of quantum numbers represents the highest energy level in an atom?
n = 4, l = 0, m = 0, s = +
n = 3, l = 1, m = 1, s = +
n = 3, l = 2, m = -2, s = +
n = 3, l = 0, m = 0, s = +
If the energies of the two photons in the ratio of 3 : 2, their wavelength will be in the ratio of
2 : 3
9 : 4
3 : 2
1 : 2
In an alkaline medium, glycine predominantly exists as/ in a/ an
cation
anion
Zwitter ion
covalent form
The "spin only'' magnetic moment of Ni2+ in aqueous solution would be [At. no. of Ni = 28]
BM
BM
BM
BM
C.
BM
Ni2+ in aqueous solution refers to [Ni(H2O)6]2+. Ni2+ has following electronic orientation.
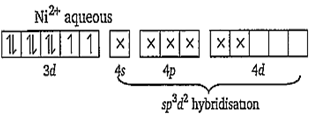
Number of unpaired electrons = 2
Spin only magnetic moment =
= BM
The correct set of four quantum numbers for the outermost electron of sodium (Z = 11) is
3, 1, 1,
3, 2, 1,
3, 0, 0,
3, 1, 0,
The statement that is not correct is
Angular quantum number signifies the shape of the orbital
Energies of stationary states in hydrogen like atoms is inversely proportional to the square of the principal quantum number
Total number of nodes for 3s-orbital is three
The radius of the first orbit of He is half that of the first orbit of hydrogen atom
The two electrons have the following set of quantum numbers, P =3,2, -2 +1/2, Q =3,0,0 + 1/2. Which of the following statement is true?
P and Q have same energy
P has greater energy than Q
P has lesser energy than Q
P and Q represent same electron
