 Short Answer Type
Short Answer Type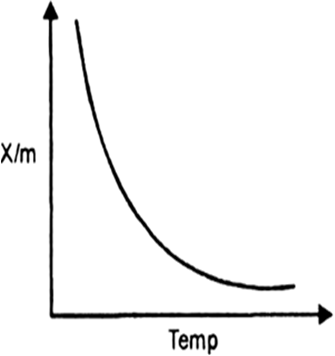
Freundlich adsorption isotherm: Freundlich gave an empirical relationship between the quantity of gas adsorbed by a unit mass of solid adsorbent and pressure at a particular temperature. The
relationship can be expressed by the following equation:![]()
where x is the mass of the gas adsorbed on mass m of the adsorbent at pressure P, k and n are constants which depend on the nature of the adsorbent and the gas at a particular
temperature. The relationship is generally represented in the form of a curve where the mass of the gas adsorbed per gram of the adsorbent is plotted against pressure.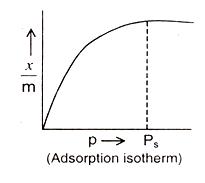
At low pressures: x/m varies linearly with p
![]()
At high pressures :x/m is independent of p
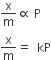
At intermediate pressures:The variation of x/m vs p can be expressed as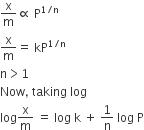
Comparing the above-given equation with the equation of a straight line
y = mx + c
we know that, if we plot log p vs log x/m, we would get a straight line with slope equal to 1/n and intercept log k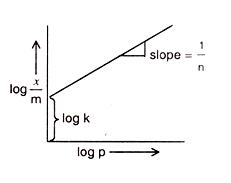
Since adsorption is always an exothermic process, therefore, increase in temperature should decrease the amount adsorbed.
