 Short Answer Type
Short Answer Type
(i) What is the charge of AgI colloidal particles in the two test tube (A) and (B).
(ii) Give reason for the origin of change.
 Long Answer Type
Long Answer Type Short Answer Type
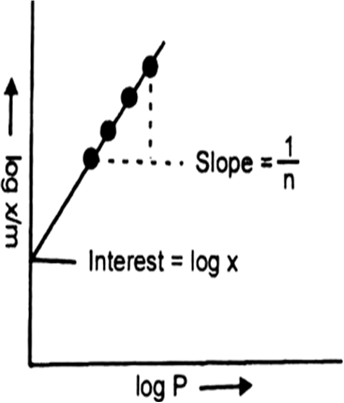
Short Answer Type(i) How are the values of k and n determined experimentally?
(ii) Explain why the value of n should be equal to or greater than one?
 Long Answer Type
Long Answer TypeExplain the following observations:
(a) Ferric hydroxide sol gets coagulated on addition of sodium chloride solution.
(b) Cottrell’s smoke precipitator is fitted at the mouth of the chimney used in factories.
(c) Physical adsorption is multi layered, while chemisorption non layered.
 Short Answer Type
Short Answer Type