 Short Answer Type
Short Answer Type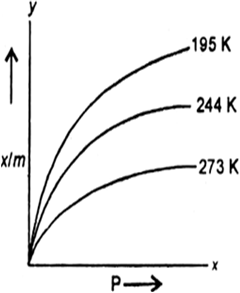
(i) temperature increases at constant pressure.
(ii) pressure increases at constant temperature.
 Long Answer Type
Long Answer TypeAnswer:
Purification of colloidal solutions:
Dialysis: It is the process of separating colloidal particles from those of crystalloids by diffusing the mixture through a parchment or animal membrane. Colloidal particles do not pass through while those of crystalloids do. The impure sol is filled into a cellophane bag, which is suspended in a vessel containing distilled water. The crystalloid particles pass through while colloidal particles do not.
Ultra filtration: It is the process of purifying a colloidal particle by treatment with a filter paper treated with colloidion which reduces the pore size of the filter paper. The sol is poured over the ultrafilter which permits the electrolytic solution to pass but retains the colloidal particles.
