 Multiple Choice Questions
Multiple Choice QuestionsIf x is amount of adsorbate and m is amount of adsorbent, which of the following relations is not related to adsorption process?
Which of the following represents physical adsorption?



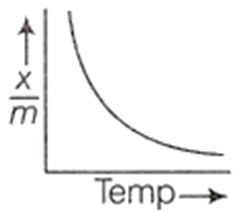
D.

The physical adsorption isobar shows a decrease in x/m throughout with rise in temperature. Therefore, the last option is correct.
In the presence of a catalyst, the heat evolved or absorbed during the reaction
increases
decreases
remains unchanged
may increase or decrease
Gold number is associated with
amount of gold
protective colloids
purple of cassius
electrophoresis
Which one of the following forms micelles in aqueous solution above certain concentration?
Urea
Dodecyl trimethyl ammonium chloride
Pyridinium chloride
Glucose
Migration of colloidal particles towards the oppositely charged electrode is called:
Electrosmosis
Electrophoresis
Coagulation
Electrodispersion
The variation of adsorption of N, on charcoal with pressure at different constant temperature can be represented by :

The correct increasing order of temperature
T1 , T2 and T3 is :
T1 < T2 < T3
T2 < T3 < T1
T3 < T2 < T1
T2 < T1 < T3
Which of the following statement is not correct?
Adsorption energy for chemical adsorption is generally greater than that of physical adsorption
Physical adsorption is due to van der Waal forces
Physical adsorption decreases at high temperature and low pressure
Physical adsorption is irreversible
