 Short Answer Type
Short Answer TypeGive Possible reason for the fact that the radii of Mn2+ ions of the first row transition metals. Ti2+ (Z = 22) to Cu2+ (Z = 29) decrease with increasing atomic number.
Explain the catalytic action of Fe(III) between the reaction of I– and persulphate ion S2O82–.
What can be inferred from the magnetic moment values of the following complex species?
|
Example |
Magnetic Moment (BM) |
|
K4[Mn(CN)6] |
2.2 |
|
[Fe(H2O)6]2+ |
5.3 |
|
K3 [Mn Cl4] |
5.9 |
Explain the structures of dichromate and chromate ion.
The chromate ion (CrO42–) has a tetrahedral shape, whereas dichromate ion has two tetrahedral units with one common oxygen having Cr—O—Cr bond angle of 126°.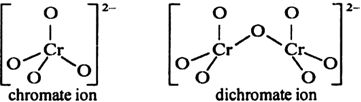
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeWhy are transition metal fluorides ionic in nature, whereas bromides and chlorides are covalent in nature.
Why are the ionization enthalpies of 5d elements greater than those of 3d and 4d elements?
