 Short Answer Type
Short Answer TypeComplete the following reaction equation:
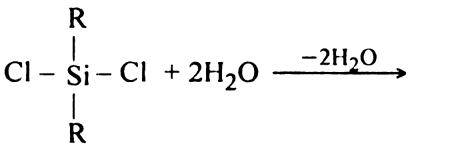
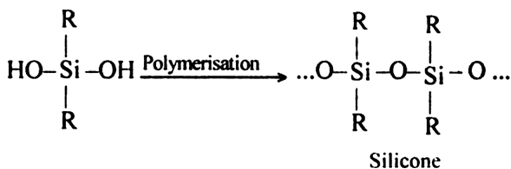
 Long Answer Type
Long Answer TypeDiscuss the characteristics of group 13 elements in terms of:
(i) Atomic and ionic radii
(ii) ionisation enthalpy
(iii) Density, melting point and boiling points.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeExplain the following:
(i) Boron is trivalent
(ii) Boron and aluminium tend to form covalent compounds.
Discuss the pattern of variation in the oxidation states of B(Boron) to Tl(Thallium).
Or
What is inert pair effect? Illustrate it with reference to Boron family.
 Short Answer Type
Short Answer TypeStandard electrode potential values  and that of
and that of  is +1.26 V. Predict about the formation of M3+ ion in solution and compare the electropositive character of the two metals.
is +1.26 V. Predict about the formation of M3+ ion in solution and compare the electropositive character of the two metals.
