 Short Answer Type
Short Answer TypeExplain the following:
(i) Boron has high melting and boiling points.
(ii) Aluminium is a good reducing agent.
 Long Answer Type
Long Answer TypeDiscuss the characteristics of Group-13 elements in terms of metallic character and their tendency to exhibit inert pair effect.
 Short Answer Type
Short Answer TypeBoron chloride exists a monomer while in the same group anhydrous, AlCl3 exists as a dimer?
Boron trichloride is a planar molecule and three covalent bond results due to sp2- hybridisation. 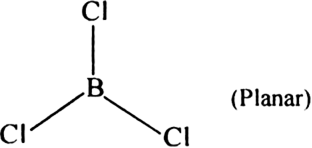
BCl3 does not form a dimer. On the other hand, aluminium trichloride exists in the dimeric state (Al2Cl6).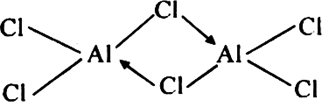
In the dimeric state, each aluminium atom accepts a pair of electrons from the chlorine atom of another aluminium chloride molecule and thereby acquires an octet of electrons. In other words AlCl3 achieves stability by forming a dimer.
White fumes appear around the bottle of anhydrous aluminium chloride.
Or
Why aluminium chloride in air?
 Long Answer Type
Long Answer TypeSuggest a reason why the B - F bond lengths in BF3 (130 pm) and  (143 pm) differ?
(143 pm) differ?
Or
Why B - F bond length in BF3 is smaller than the expected value?
 Short Answer Type
Short Answer Type