 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeHow does aluminium react with:
(i) boiling water.
(ii) concentrated H2SO4
(iii) dilute nitric acid
(iv) concentrated HCl
(v) NaOH?
(i) Action with boiling water. It decomposes boiling water to liberate hydrogen.
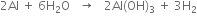
(ii) Action with concentrated H2SO4. It reacts with hot concentrated sulphuric acid to liberate sulphur dioxide.

(iii) Action with dilute nitric acid. Aluminium reacts with dilute nitric acid to form aluminium nitrate and ammonium nitrate.

(iv) Action with concentrated HCl. Aluminium dissolves in moderately concentrated hydrochloric acid to form (hydrated) chloride.
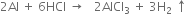
(v) Action with NaOH. It dissolves in hot sodium hydroxide solution to form sodium meta-aluminate.

 Short Answer Type
Short Answer Type