 Short Answer Type
Short Answer TypeExplain the following phenomenon by means of balanced equations:
(i) When exhaling is made through a tube passing into a solution of lime water, solution becomes turgid.
(ii) The turbidity of the above solution.
(iii) eventually disappears when continued exhaling is made through it.
 Long Answer Type
Long Answer TypeExplain why silicon tetrachloride is hydrolyzed but carbon tetrachloride is not hydrolysed.
 Short Answer Type
Short Answer TypeContrast the structure and properties of CO2 and SiO2.
| Carbon dioxide(CO2) | Silica (SiO2) |
| 1. It is a monomeric linear gaseous molecule as O =C = O | 1. It is a solid network having a three-dimensional structure in which each silicon atom is tetrahedrally surrounded by four oxygen atoms. |
| 2. It is soluble in water. | 2. It is insoluble in water. |
3. On reduction with coke (carbon), it forms carbon monoxide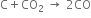 |
3. On reduction with carbon in an electric furnace, it produces silicon carbide.  |
