413.
What type of hybridisation is associated with N in NH3,? What is the expected bond angle in NH3?
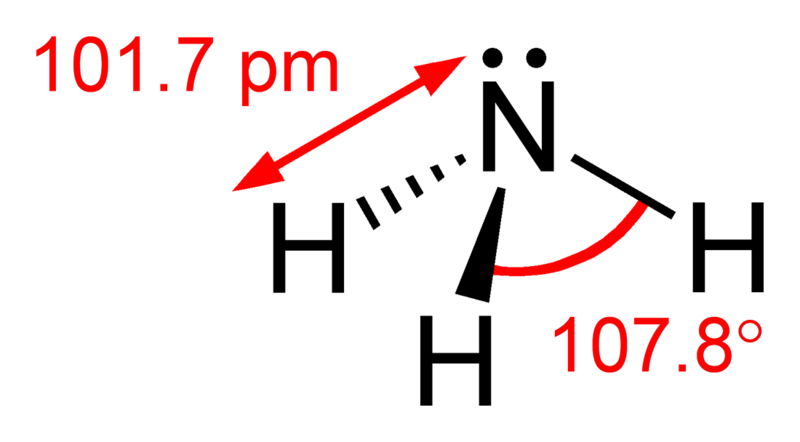
In the ammonia molecule (NH3), 2s and 2p orbitals create four sp3 hybrid orbital, one of which is occupied by a lone pair of electrons
sp3 hybridisation, expected bond angle = 109°28', actual bond angle = 107°.
250 Views
 Short Answer Type
Short Answer Type
