Multiple Choice Questions
Multiple Choice QuestionsWhich of the following equations correctly represents the standard heat of formation (H) of methane ?
C (diamond) + 4H (g) → CH4 (g)
C (diamond) + 2H2 (g) → CH4 (g)
C (graphite) + 2H2 (g) → CH4 (g)
C (graphite) + 4H (g) → CH4 (g)
Graphite is a soft solid lubricant extremely difficult to melt. The reason for this anomalous behaviour is that, graphite
is a non-crystalline substance
is an allotropic form of diamond
has molecules of variable molecular masses like polymers
has carbon atoms arranged in large plates of rings of strongly bound carbon atoms with weak interplate bonds
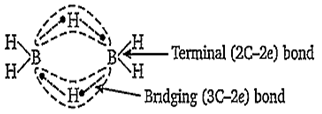
The structure of diborane (B2H6) contains
four 2C-2e- bonds and four 3C-2e- bonds
two 2C-2e- bonds and two 3C-3e- bonds
two 2C-2e- bonds and four 3C-2e- bonds
four 2C-2e- bonds and two 3C-2e- bonds
D.
four 2C-2e- bonds and two 3C-2e- bonds
The structure of diborane is-