 Multiple Choice Questions
Multiple Choice QuestionsIn silica (SiO2), each silicon atom is bonded to
two oxygen atoms
four oxygen atoms
one silicon and two oxygen atoms
one silicon and four oxygen atoms
B.
four oxygen atoms
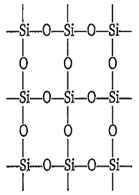
The giant molecule of silicon dioxide or silica (SiO2) consists of continuous lattice of silicon and oxygen connected by covalent bonds. In silica each silicon atom is tetrahedrally surrounded by four oxygen atoms. Thus, there are no discrete SiO2 units. SiO2 is a network solid.
In silica, silicon has large size, so the 3p-orbitals of Si do not overlap effectively with 2p-orbitals of oxygen. Therefore, Si = O are not formed. The tetravalency of Si is satisfied by the formation of Si-O bonds, thus it is surrounded by four oxygen atoms.
The magnetic nature of boron molecule is same as the magnetic nature of
nitrogen molecule
carbon molecule
unipositive nitrogen molecule
oxide ion
Which of the following is not a true alum?
Na2SO4.Al2(SO4)3.24H2O
MgSO4.Cr2(SO4)3.24H2O
K2SO4.Cr2(SO4)3.24H2O
(NH4)2SO4.Fe(SO4)3.24H2O
The densities of graphite and diamond at 298 K are 2.25 and 3.31 g cm-3 respectively. If the standard free energy difference is 1895 J mol-1, the pressure at which graphite will be transformed into diamond is
9.92 × 108 Pa
9.92 × 107 Pa
9.92 × 106 Pa
None of these
Which one of the following elements does not form triiodide on reacting with iodine?
B
Ti
Al
Ga
