 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWhat happens when:
(i) Lithium reacts with air
(ii) Lithium reacts with water
(iii) Lithium reacts with halogen
(iv) Lithium reacts with acids?
 Short Answer Type
Short Answer TypeAccount for the following:
(i) Lithium can not form monovalent cation (Li+) easily.
(ii) Lithium iodide is more covalent than lithium fluoride.
 Long Answer Type
Long Answer TypeWhat difficulities arise in the extraction of sodium? How these difficulties are overcome?
Discuss Down's process for the isolation of sodium.
In this method, sodium is prepared by the electrolysis of fused anhydrous sodium chloride. Some calcium chloride and potassium fluoride are added to it. It lowers the melting point of sodium chloride from 1085 to 873K. The electrolysis is carried out in an iron box lined with fire bricks known as Down’s cell.
It consists of a graphite anode projected up through the bottom of the cell which is surrounded by a cylindrical iron cathode. The anode and cathode are separated by a wire gauze shell through which molten sodium chloride can easily pass but melted sodium cannot. The cell is also produced with a storage drum for receiving molten metal, an outlet for the removal of chlorine gas at the top.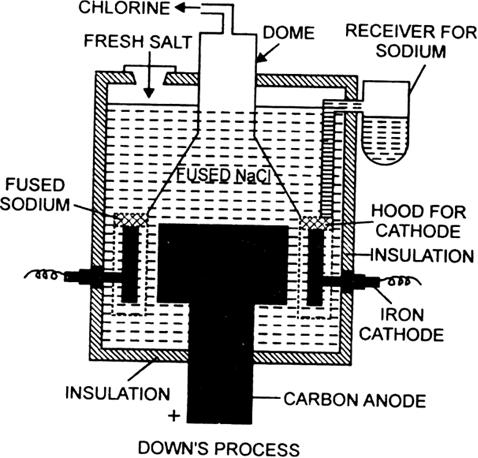
On electrolysis, chlorine is liberated at the anode which escapes through the iron hood at the top. Sodium is liberated at the cathode and remains in the wire gauze shell. The level of molten sodium rises and it overflows into the receiver. The following reactions take place: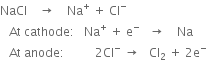
Sodium metal obtained by this method is about 99.5% pure.
 Short Answer Type
Short Answer TypeWhat happens when:
(i) sodium reacts with hydrogen halide,
(ii) sodium reacts with acetylene,
(iii) sodium is heated with hydrogen and
(iv) sodium is treated with mercury ?
