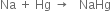Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWhat happens when:
(i) Lithium reacts with air
(ii) Lithium reacts with water
(iii) Lithium reacts with halogen
(iv) Lithium reacts with acids?
 Short Answer Type
Short Answer TypeAccount for the following:
(i) Lithium can not form monovalent cation (Li+) easily.
(ii) Lithium iodide is more covalent than lithium fluoride.
 Long Answer Type
Long Answer TypeWhat difficulities arise in the extraction of sodium? How these difficulties are overcome?
 Short Answer Type
Short Answer TypeWhat happens when:
(i) sodium reacts with hydrogen halide,
(ii) sodium reacts with acetylene,
(iii) sodium is heated with hydrogen and
(iv) sodium is treated with mercury ?
(i) When sodium is treated with hydrogen halide, hydrogen gas is evolved.
   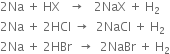
(ii) Sodium reacts with acetylene to form sodium acetylide.
                    
(iii) When sodium is heated with hydrogen, sodium hydride is produced. 
         
(iv) When sodium is treated with mercury, sodium amalgam is formed as the product.