 Long Answer Type
Long Answer TypeWhat happens when:
(i) sodium metal is dropped in water?
(ii) sodium metal is heated in free supply of air?
(iii) sodium peroxide dissolves in water?
 Short Answer Type
Short Answer TypeAccount for the following:
(i) Sodium imparts colour to the flame.
(ii) Sodium acts as a strong reducing agent.
(i) Due to low ionisation energy, when sodium metal or its salt is heated in a flame, the valence electron is excited to higher energy level. When this excited electron returns to the ground state, the absorbed energy is emitted in the visible region of the electromagnetic spectrum and hence the flame appears coloured.
(ii) The reducing character of sodium is due to its low ionisation energy as a result of which it can easily lose its valence electrons and act as strong reducing agent. For example,
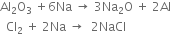
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type