 Short Answer Type
Short Answer TypeWhat is the action of heat on Na2CO3.10H2O?
On heating below 373K, it loses 9 molecules of water of crystallisation to form monohydrate (Na2CO3.H2O). On heating above 373K, the monohydrate changes to an anhydrous white powder called soda ash but does not decompose further.
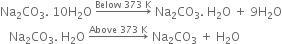
What happens when:
(i) sodium carbonate reacts with the milk of lime.
(ii) sodium carbonate is added to water.
(iii) sodium carbonate reacts with a dilute mineral acid?
 Long Answer Type
Long Answer TypeHow is sodium hydroxide manufactured? Discuss in brief the details of the process.
Or
With the help of a diagram, show the reactions at the cathode and anode in the manufacture of sodium hydroxide by the Castner - Kellner process.
 Short Answer Type
Short Answer TypeExplain what happens when:
(i) Sodium hydrogen carbonate is heated
(ii) Sodium amalgam reacts with water
(iii) Fused sodium metal reacts with ammonia?
Explain the significance of sodium, potassium, calcium and magnesium in biological fluids ?
