 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWhat is meant by the diagonal relationship of elements? Discuss the diagonal relationship of beryllium with aluminium.
Or
Beryllium exhibits some similarities with aluminium. Point out three such properties.
Discuss the diagonal realtionship of Be and Al with regard to:
(i) action of alkali (ii) structure of chlorides.
(i) The action of alkali: Both the metals dissolve in strong alkalies to form soluble complexes and liberate hydrogen.
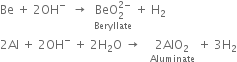
(ii) The structure of chlorides: Structure of BeCl2: In the solid state, it exists as polymeric chain structure. In vapour state, beryllium chloride exists in the dimeric form which decomposes at 1200K into monomeric form. 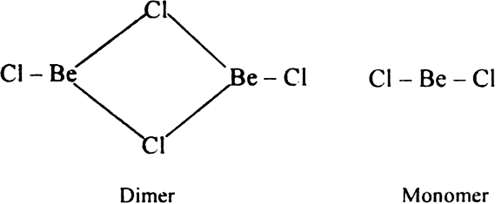
The structure of AlCl3: Aluminium chloride exists as a dimer (Al2Cl6). In this dimeric structure, each aluminium atom forms one co-ordinate bond by accepting a lone pair of electrons from the chlorine atoms covalently bonded to the other aluminium atom.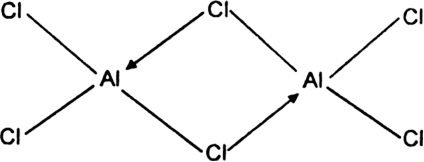
Both the aluminium atoms complete their octet.
 Short Answer Type
Short Answer TypeGive any three points of similarities between beryllium and aluminium and two points of dissimilarities between beryllium and barium ?
 Long Answer Type
Long Answer TypeHow does magnesium occur in nature? How is magnesium obtained by electrolysis method ?
What happens when:
(i) magnesium is heated with water.
(ii) magnesium is heated in an atmosphere of carbon dioxide,
(iii) magnesium is treated with dilute sulphuric acid and
(iv) magnesium is treated with nitrogen?
