 Long Answer Type
Long Answer TypeHow is slaked lime prepared? What are its properties and uses?
Slaked lime is prepared by the following methods:
(i) From quick lime: By treating quicklime with water, slaked lime is formed.

When water is added to quicklime, a huge amount of heat is produced along with the hissing sound.
(ii) From calcium chloride: By treating calcium chloride with caustic soda, slaked lime is formed.
Properties:
(i) Slaked lime is a white amorphous powder.
(ii) A suspension of slaked lime in water is called milk of lime.
(iii) The aqueous layer which is decanted from the precipitated calcium hydroxide is called lime water.
(iv) On passing carbon dioxide through lime water, the lime water turns milky due to the formation of insoluble calcium carbonate. 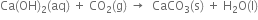
If carbon dioxide is passed in excess, a clear solution is again obtained. This is because the insoluble calcium carbonate changes into soluble calcium bicarbonate.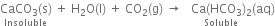
If the clear solution is heated, it again turns milky due to the decomposition of calcium bicarbonate into calcium carbonate. 
(v) Slaked lime reacts with chlorine to form bleaching powder.
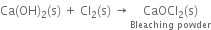
Uses. It is used:
(i) for the preparation of ammonia
(ii) for white washing
(iii) in the purification of sugar
(iv) in the softening of hard water.
 Short Answer Type
Short Answer TypeHow is quick lime prepared on a commercial scale? How is it converted into slaked lime?
 Long Answer Type
Long Answer TypeWhat is the effect of heat on the following compounds? (Write equations for the reactions):
(i) Calcium Carbonate
(ii) Magnesium chloride hexahydrate
(iii) Gypsum
(iv) Magnesium sulphate heptahydrate
 Short Answer Type
Short Answer Type