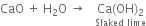Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeHow is quick lime prepared on a commercial scale? How is it converted into slaked lime?
 Long Answer Type
Long Answer TypeWhat is the effect of heat on the following compounds? (Write equations for the reactions):
(i) Calcium Carbonate
(ii) Magnesium chloride hexahydrate
(iii) Gypsum
(iv) Magnesium sulphate heptahydrate
 Short Answer Type
Short Answer TypeGive one method of preparing quick lime. What happens when rain water falls on it?
Calcium oxide is called quicklime. It is prepared by heating the lime stone in a rotatory kiln at 1070-1270 K.
CO2 formed escapes and hence the above equilibrium shifts towards the formation of calcium oxide.
When rain-water falls on quick lime, slaked lime is formed.