 Short Answer Type
Short Answer TypeStarting with CaCO3, write balanced chemical equation showing the preparation of:
(i) CaO (ii) CaSO4 (iii) Ca(OH)2 (iv) Ca(HCO3)2 solution.
 Long Answer Type
Long Answer TypeDescribe two important uses of the following;
(a) Caustic soda (b) Sodium carbonate (iii) Quick lime.
What happens when,
(i) magnesium is burnt in air
(ii) quick- lime is heated with silica
(iii) chlorine reacts with slaked lime
(iv) calcium nitrate is heated?

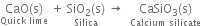


Discuss the composition and manufacturing details of cement.
Or
Mention the main constituents of Portland cement.
 Short Answer Type
Short Answer TypeContrast the action of heat on the following and explain your answer:
(i) Na2CO3 and CaCO3
(ii) MgCl2.6H2O and CaCl2.6H2O
(iii) Ca(NO3)2 and NaNO3.
Discuss the general characteristics and gradation in properties of alkaline earth metals.
Compare the alkali metals and alkaline earth metals with respect to (i) ionisation enthalpy (ii) basicity of oxides and (iii) solubility of hydroxides.
