 Long Answer Type
Long Answer TypeExplain why can alkali and alkaline earth metals not be obtained by chemical reduction methods?
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeCompare the solubility and thermal stability of the following compounds of the alkali metals with those of the alkaline earth metals. (a) Nitrates (b) Carbonates (c) Sulphates.
Starting with sodium chloride how would you proceed to prepare (i) sodium metal (ii) sodium hydroxide (iii) sodium peroxide (iv) sodium carbonate?
Na is prepared from NaCl by the following method: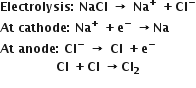
2) Sodium hydroxide is prepared by carrying out the electrolysis of the aqueous solution of sodium chloride either in Nelson' cell or Castner Kellner cell.
Preparation of sodium peroxide: Sodium chloride is first converted in sodium by electrolytic reduction. The metals are then heated with an excess of oxygen at about 573K in an atmosphere free from moisture and carbon dioxide to form sodium peroxide.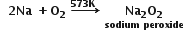
3) sodium carbonate is prepared from an aqueous solution of NaCl by Solvay process. In this process, CO2 is passed through NaCl solution saturated with ammonia when the following reaction occurs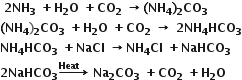 :
:
Describe the importance of the following: (i) limestone (ii) cement (iii) plaster of paris.
 Multiple Choice Questions
Multiple Choice QuestionsAmongst the following, select the element having highest ionization enthalpy.
Sodium
Potassium
Beryllium
Magnesium
The main oxides formed on combustion of Li, Na and K in excess of air are, respectively:
LiO2, Na2O2 and K2O
LiO2, Na2O2 and KO2
LiO, Na2O2 and KO2
LiO, Na2O2 and KO2
Which one of the following alkaline earth metal sulphates has its hydration enthalpy greater than its lattice enthalpy?
CaSO4
BeSO4
BaSO4
BaSO4
The correct statement for the molecule, CsI3 is
It is a covalent molecules
It contains Cs+ and I3-
It contains Cs3+ and I- ions
It contains Cs3+ and I- ions
Which of the following on thermal decomposition yields a basic as well as an acidic oxide?
NaNO3
KClO3
CaCO3
CaCO3
