 Short Answer Type
Short Answer TypeCalculate the difference between heats of reaction at constant pressure and constant volume for the reaction:![]() at
at ![]()
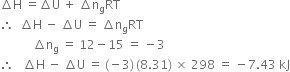
What is the mode of transference of energy, when petrol is subjected to combustion in an internal combustion engine ?
