 Short Answer Type
Short Answer TypeOut of the following different forms of oxygen, which will have the standard enthalpy of the formation to be 0.0 kJ; O, O2(g), O3(g), O2(l)?
Why the heat of neutralisation is less than 57.1 kJ if either the acid or the base or both are weak?
Will the heat evolved be same in the following two cases?![]()
![]()
If not, in which case it will be greater and why?
Which of the following is/are exothermic and which is/are endothermic/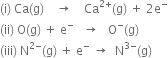
(i) Endothermic (ionisation enthalpy is required).
(ii) Exothermic (first electron gain enthalpy is energy released).
(iii) Endothermic (higher electron gain enthalpies are energy required)
