 Short Answer Type
Short Answer TypeWhat is the relation between enthalpy change and entropy change for a process at equilibrium?
What is the enthalpy of formation of the most stable form of an element in its standard state?
Predict the sign of entropy change for the reactions:![]()
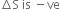
 Multiple Choice Questions
Multiple Choice QuestionsA reaction ![]() is found to have a positive entropy change. The reaction will be
is found to have a positive entropy change. The reaction will be
possible at high temperature
 Short Answer Type
Short Answer Type