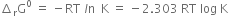Short Answer Type
Short Answer TypeHow can a chemical reaction with positive enthalpy and entropy changes be made entropy drives?
For a reaction both ∆H and T∆S are positive. Under what conditions will the reaction be spontaneous?
Write an expression that connects standard free energy change, standard enthalpy change and standard entropy change.
How is the standard free energy related to equilibrium constant?