 Short Answer Type
Short Answer TypeCompute internal energy change of a system if it
(i) absorbs 500 kJ heat and does 300 kJ work.
(ii) loses 200 kJ heat and has 450 kJ of work done on it.
(i) Here, q = 500 kJ, w = -300 kJ
According to the first law of thermodynamics,

(ii) Here, q = -200 kJ, w = 450 kJ
A sample of gas is compressed by an average pressure of 0.50 bar so as to decrease its volume from 450 cm3 to 250 cm3. During the process 6.00 J of heat flows out to surroundings. Calculate the change in internal energy of the system.
State whether each of the following will increase or decrease the total energy content of the system:
(i) heat transferred to the surroundings
(ii) work done on the system
(iii) work done by the system.
 Long Answer Type
Long Answer Type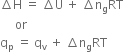
 Short Answer Type
Short Answer Type