 Long Answer Type
Long Answer TypeDefine entropy and entropy change. What are the units of entropy?
The degree of randomness or disorderliness is expressed by a thermodynamic function called entropy. It may be defined as the property of a system which measures the degree of randomness or disorderliness in the system. It is denoted by S. It is a state function. It depends only on the initial and final state of the system and not on the path followed.
If SA and SB are the entropies at state A and B, then entropy change ∆S is given by
For a chemical reaction, 
For a reversible process at equilibrium, the change in entropy may be expressed as,
Where qrev represents the total heat absorbed reversibly and isothermally at temperature T.
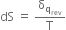
 Short Answer Type
Short Answer TypeAccount for the following:
(i) Why does real crystal have more entropy than an ideal crystal?
(ii) Why the entropy of a pure substance is taken as zero at absolute zero?
(iii) Why the entropy of the universe is continuously increasing?
Predict the change in entropy for the system in which the following physical changes occur:
(i) Dissolving cream in a cup of coffee.
(ii) The beating of an egg for an omellete.
(iii) The formation of a rain drop in a cloud.
(iv) The crystallization of a metal alloy from the molten state.
In the following changes, state whether order has increased or decreased and consequently the direction of change of entropy of the system:
In the following changes, state whether order has increased or decreased and consequently by the direction of change of entropy of the system:
Will entropy increased or decrease during boiling of an egg? Comment on the statement.
