 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeProve that in a reversible process:
∆(system) + ∆S(surroundings) = 0
Or
Prove that there is no net change in entropy in a reversible process.
Prove that in an irreversible process:
∆S(system) + ∆S(surroundings) > 0
If any part of the process is irreversible, the process as a whole is irreversible. Suppose the total heat lost by the surrounding is qirrev. This heat is absorbed by the system. However, entropy change of the system is always calculated from the heat absorbed reversibly.

This is because of the large size of the surroundings due to which heat lost (qirrev) by the surroundings can be considered as the heat lost reversibly and isothermally at temperature T.
The total entropy change for the combined system and surroundings is
We know that the work done in a reversible process is the maximum work.

Also, internal energy (U) is a state function, the value of U is same whether the process is carried out reversibly or irreversibly. Hence

From relation (4) and (5), we conclude that

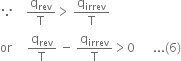
Combining the result with the result given in equation (3),

Thus, in an irreversible process, the entropy change for the combined system and the surroundings i.e. an isolated system is greater than zero i.e. an irreversible process is accompanied by a net increase of entropy.
What do you understand by:
(i) The entropy of fusion?
(ii) The entropy of vapourisation ?
 Short Answer Type
Short Answer TypeYou are given normal boiling points and standard enthalpies of vapourisation. Calculate the entropy of vapourisation of liquids listed below:
Liquid 

Calculate the entropy change of n-hexane when 1 mole of it evaporates at 341.7 K(∆Hvap = 29.0 kJ mol–1).
 Long Answer Type
Long Answer TypeWhat are the two tendencies which determine the feasibility of process? How are the two related to each other?
