 Multiple Choice Questions
Multiple Choice QuestionsCollagen is the most prevalent extracellular matrix protein. Which of the following is NOT true for collagen?
Collagen is composed of triple helix consisting of two α polypeptide chains and one β polypeptide chain wound around one another in a rose-like structure.
Glycine accounts for almost one-third of the amino acids within collagen molecule.
Ascorbate is essential for collagen formation required for hydroxylation of proline.
Individual collagen polypeptide chains are synthesized on membrane-bound ribosomes with N-terminal signal sequences for directing them to ER lumen.
An elderly person suffering from calcium deficiency was advised to take calcium-rich food and to supplement the diet with vitamin D. The absorption of calcium in the intestine with the supplementation of vitamin D. Following explanations were offered for this increased calcium absorption by vitamin D:
A. The synthesis of calbindin-D9K and calbindin-D28K in enterocytes was stimulated.
B. The number of Ca2+ - ATPase molecules in enterocytes was increased.
C. The synthesis of divalent metal transporters 1 (DMT1) in the enterocytes was stimulated.
D. The number of hephaestin in the enterocytes was increased.
Which of the above combinations is correct?
A and B
B and C
C and D
A and D
A hypothetical gene encodes a protein with the following amino acid sequence:
Phe-Pro-Thr-Ala-Val-Arg-Ser
A mutation of single nucelotide alters the amino acid sequence to
Phe-Leu-Leu-leu-Leu-Val
A second single nucleotide mutation occurs in same gene restoring back the amino acid sequence to the original.
The following statements were made regarding the nature and location of the first mutation and that of the intragenic suppressor mutation:
A. The first mutation is a deletion in the second codon.
B. the first mutation is an insertion in the second codon.
C. The intragenic suppressor mutation is an insertion in the second codon.
D. The intragenic suppressor mutation is a deletion in the third codon.
Which combination of the above statements is correct?
A and C
A and D
B and C
B and D
10mM acetate buffer (pH 4.00) is diluted one million times with distilled water (pH 7.00). pH of this diluted buffer is:
4.00
7.04
8.00
6.96
Consider the structureless oligopeptide, R-G-P-S-T-K-M-P-E-Y-G-S-T-D-Q-S-N-W-H-F-R. The number of bonds that will be cleaved by trypsin and chymotrypsin treatments separately, are:
1, 2
2, 2
2, 3
1, 3
The internal energy of a gas increases by 1J when it is compressed by a force of 1 Newton through 2 metres. The change of the system is :
1 J
-1 J
2J
-2J
for the base pairing of oligonucleotides (n = 5) at 300 K is -18 kJ mol-1. What would be the approximate value of the equilibrium constant K?
100
10
1000
1
The reaction of glutamate and ammonia to glutamine and water has a value of +14 kJ mol-1 for . This is coupled with the ATP reaction (ATP + H2O ADP + phosphate). The for this reaction is -30kJ mol-1. The (kJ mol-1) for the coupled reaction
Glutamate + NH3 + ATP Glutamine + ADP + Phosphate
under equilibrium condition is
16
-44
-16
44
A protein is composed of leucine, isoleucine, alanine, glycine, proline, one lysine, one arginine and two cysteines connected by a disulfide bond. Conformational analysis indicates that the protein has elements of helix and beta structure. The protein is most likely
a non-specific protease
not an enzyme
a lipase
a flippase
Small molecular weight compounds affect the activity of luciferase differently. The oil/ water solubility of various compounds is one property important for its effect on luciferase. The straight line in the graph was obtained by plotting the activity data with 50 different compounds. The luciferase activity, of two new derivatives of Benzene (A and B) are shown below:
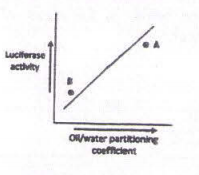
Which of the following statements is correct?
A is phosphate and B is amine.
A is methyl and B is amine.
A is methyl and B is propyl.
A is amine and B is phosphate.
