Multiple Choice Questions
Multiple Choice QuestionsDuring episodes of anoxia in plants, pyruvate produced in glycolysis is initially fermented to lactate. During later stage, there is an increase in the fermentation to ethanol and decrease in the fermentation to lactate, a phenomena which helps plants survive anoxia. Which of the following statements is correct about this change of fermentation flux from lactate towards ethanol?
The cytosolic pH increases, thus activating both lactate dehydrogenase and pyruvate decarboxylase activity
The cytosolic pH increase, thus inhibiting lactate dehydrogenase activity and activating pyruvate decarboxylase activity
The cytosolic pH decreases, thus activating both lactate dehydrogenase and pyruvate decarboxylase activity
The cytosolic pH decreases, thus inhibiting lactate dehydrogenase and activating pyruvate decarboxylase activity.
The MALDI spectrum of a peptide shows a peak at m/z corresponding to 3600. When the ESI spectrum is recorded, peaks at m/z corresponding to 721,904 and 1801 were obtained. When the MALDI MS/MS spectrum was recorded, large number of peaks with m/z less than 3600 were observed. The spectral data indicate that the peptide is
Highly impure
Pure with molecular mass of 3600 and partial sequence of the peptide can be determined
Highly unstable and degrades rapidly
Degraded under condition employed for recording ESI spectrum
Proteins in the cells can be visualized by the following methods:
A. Express the gene (coding for the said protein) as a fusion with the green fluorescence protein (GFP) and directly visualize under a fluorescence microscope.
B. Express the gene (coding for the said protein) as a fusion with the β‐ galactosidase gene (lac Z) and directly visualize under a phase contrast bright field microscope.
C. A fluorescence tagged antibody raised against the said protein could be used for visualization in a fluorescence microscope.
D. Over express the protein and directly visualize it under a scanning electron microscope.
Which of the following methods you would choose to visualize a protein in a living cell?
A only
A and C only
A and B only
D only
A reference material is required to be prepared with 4 ppm calcium. The amount of CaCO3 (molecular weight = 100) required to prepare 1000 g of such a reference material is
10 µg
4 µg
4 mg
10 mg
The variation of solubilities of two compounds X and Y in water with temperature is depicted below. Which of the following statements is true?
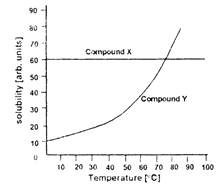
Solubility of Y is less than that of X.
Solubility of X varies with temperature.
Solubilites of X and Y are the same at 75°C.
Solubilities of X and Y are independent of temperature.
Magnesium powder, placed in an air-conditioned glass container at 1.0 bar, is burnt by focusing sunlight. Part of the magnesium burns off, and some is left behind. The pressure of the air in the container after it has returned to room temperature is approximately.
1.0 bar
0.2 bar
1.2 bar
0.8 bar
When a magnet is made of fall free in air, it falls with an acceleration of 9.8 m s-2. But when it is made to fall through a long aluminum cylinder, its acceleration decreases, because
A part of the gravitational potential energy is lost in heating the magnet.
A part of the gravitational potential energy is los in heating the cylinder.
The said experiment was done in the magnetic northern hemisphere.
The cylinder shields the gravitational force.
The conductance of a potassium chloride solution is measure using the arrangement depicted below. The specific conductivity of the solution in Sm-1, when there is no deflection in the galvanometer, is
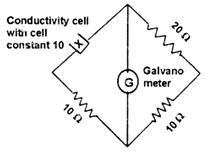
1.0
0.5
2.0
1.5
What is the half-life of the radio isotope whose activity profile is shown below?
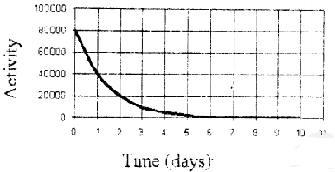
1 day
3 days
2 days
4 days
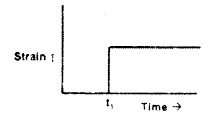
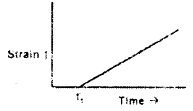
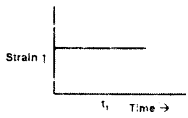
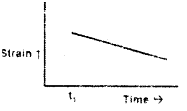
For an elastic material, strain is proportional to stress. A constant stress is applied at time t1. Which of the following plots characterizes the strain in that material?