 Multiple Choice Questions
Multiple Choice QuestionsIn the chemical reaction, CH3CH2NH2 + CHCl3 + 3KOH → (A) + (B) + 3H2O, the compounds (A) and (B) are respectively
C2H5CN and 3KCl
CH3CH2CONH2 and 3KCl
C2H5NC and K2CO3
C2H5NC and K2CO3
Which one of the following is the strongest base in aqueous solution?
Trimethylamine
Aniline
Dimethylamine
Dimethylamine
Which one of the following methods is neither meant for the synthesis nor for separation of amines?
Hinsberg method
Hofmann method
Wurtz reaction
Wurtz reaction
Amongst the following the most basic compound is
benzylamine
aniline
acetanilide
acetanilide
Which one the following does not have sp2 hybridized carbon?
Acetone
Acetamide
Acetonitrile
Acetonitrile
Consider the acidity of the carboxylic acids
PhCOOH
p – NO2C6H4COOH
o – NO2C6H4COOH
o – NO2C6H4COOH
Which of the following compounds will be suitable for Kjeldah1’s method for nitrogen estimation?

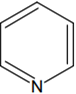


The increasing order of basicity of the following compounds is:

(d) < (b) < (a) < (c)
(a) < (b) < (c) < (d)
(b) < (a) < (c) < (d)
(b) < (a) < (d) < (c)
D.
(b) < (a) < (d) < (c)
Order of base nature depends on electron donation tendency.
It compounds (b) nitrogen is sp2 hybridized so least basic among all given compound
Compound (c) is a very strong nitrogeneous organic base as a lone pair of one nitrogen delocalize in resonance and make another nitrogen negatively charged and conjugate acid have two equivalent resonating structure.
Thus it is most basic in given compound.
(d) is secondary amin more than (a) which is a primary amine.
The compound that does not produce nitrogen gas by the thermal decomposition is
(NH4)2SO4
Ba(N3)2
(NH4)2Cr2O7
NH4NO2
