 Multiple Choice Questions
Multiple Choice QuestionsWhich one of the following is the strongest base ?
2,4,6-trinitroaniline
2,4,6-trinitro-N,N-dimethyl aniline
N, N-dimethyl aniline
Anline
Hinsberg's reagent is
benzene sulphonyl chloride
benzene sulphonic acid
phenyl isocyanide
benzene sulphonamide
The given reaction is called as

Schmidt rearrangement
Curtius rearrangement
Hofmann rearrangement
Lossen rearrangement
Correct basicity of the following compounds are
I. Aniline
II. Pyridine
III. Pyrrole
IV. Guanidine
I >II >III >IV
III >I >II >IV
IV >II >I >III
II >IV>III >I
One amine is more basic than ammonia and the other is less basic than ammonia. The two amines are respectively
N-methyl ethanamine and N, N-dimethyl ethanamine
aniline and N-methyl aniline
N-methyl aniline and aniline
N, N-dimethyl aniline and benzenamine
A.
N-methyl ethanamine and N, N-dimethyl ethanamine
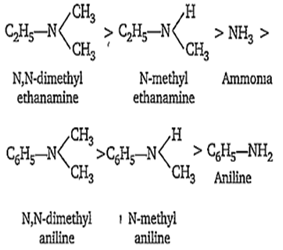
Three classes of amine, i.e. 1°, 2° and 3° amine are stronger bases than ammonia. It is due to the reason that alkyl groups are electron donating groups. As a result, the electron density on the nitrogen atom increases and thus, they can donate the lone pair of electrons more easily than ammonia. Thus, the basicity of amines should decrease in the order 3° amine > 2° amine > 1° amine > ammonia.
Aromatic amines are far less basic than ammonia. This is because due to resonance in aniline, the lone pair of electrons on the nitrogen atom gets delocalised over the benzene ring and thus is less easily available for protonation.
When the hydrogen atom of the amino group in aniline are replaced by electron donating alkyl groups, the basicity of resultant arylamines increases. e.g., N-methylaniline is a stronger base than aniline and N, N-dimethyl aniline is even stronger than N-methylaniline. However, they are not stronger bases than ammonia. Thus, the basicity of N-substituted anilines relative to aniline follows the sequence
NH3 > > C6H5-NH2
Thus, the overall decreasing order of basicities of different amines is
One of the isomer of C4H11N is optically active. It must be a
primary amine
secondary amine
tertiary amine
all isomers are optically inactive
Which of the following compounds cannot be identified by carbylamine test?
CHCl3
C6H5-NH-C6H5
C6H5NH2
CH3CH2NH2
Acetamide and ethylamine can be distinguished ,by reacting with
Br2 water
acidic KMnO4
aq HCl and heat
aq NaOH and heat
The basicity of aniline is less than that of cyclohexylamine. This is due to :
+R-effect of-NH2 group
-I effect of -NH2 group
-R effect of-NH2 group
hyperconjugation effect
Which of the following would undergo Hofmann reaction to give a primary amine?
R-CO-Cl
RCONHCH3
RCONH2
RCOOR
