 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following compounds will give red colour on Lassaigne test?
NaCNS
NH2CSNH2 (thiourea)
p-NH2C6H4SO3H (p- aminobenzene sulphonic acid)
all of the above
A positive carbylamine test is given by
I. N, N-dimethylaniline
II. 2, 4-dimethylamine
III. N-methyl-o-methylaniline
IV. p-methylbenzyl amine
(II) and (IV)
(I) and (IV)
(II) and (III)
(I) and (II)
The IUPAC name of the following compound is
![]()
2-carbamoylhexanal
2-carbamoylhex-3-enal
2-methyl-6-oxohex-3-enamide
6-keto-2-methyl hexamide
Cyclohexylamine and aniline can be distinguished by
Hinsberg test
Carbylamine test
Lassaigne test
Azo dye test
Nitration of phenyl benzoate yields the product
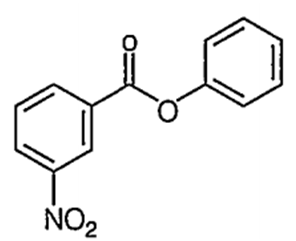
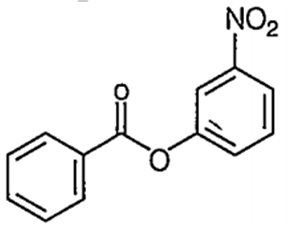
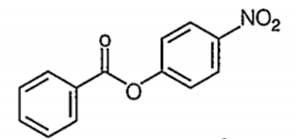
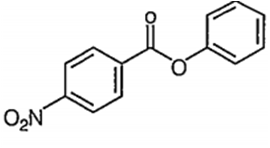
C.

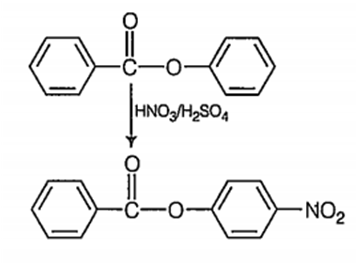
It is an electrophilic aromatic substitution reaction. Because the COO- acts as an electron wthdrawing group to one phenyl group and a moderate electron donating group to the other phenyl group the nitration wll occur on the phenyl group where COO- is acting as an
electron donating group.
The major product of the nitration will have the -NO2 in the para position because the ortho positions partially blocked by the large COPh group.
