 Multiple Choice Questions
Multiple Choice QuestionsThe decreasing values of bond angles from NH3 (106o ) to SbH3 (101o ) down group-15 of the periodic table is due to
increasing bp-bp repulsion
increasing p-orbital character in sp3
decreasing lp-bp repulsion
decreasing lp-bp repulsion
The number and type of bonds between two carbon atoms in calcium carbide are
One sigma, one pi
One sigma, two pi
Two sigma, one pi
Two sigma, one pi
Of the following sets which one does NOT contain isoelectronic species?
PO-34,SO4-2, ClO4-
CN-, N2, C2-2
SO3-2, CO3-2,NO3-
SO3-2, CO3-2,NO3-
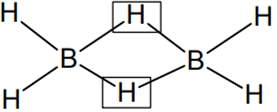
The structure of diborane (B2H6) contains
four 2c-2e bonds and two 3c-2e bonds
two 2c-2e bonds and four 3c-2e bonds
two 2c-2e bonds and two 3c-3e bonds
two 2c-2e bonds and two 3c-3e bonds
A.
four 2c-2e bonds and two 3c-2e bonds
The correct order of bond angles (smallest first) in H2S, NH3, BF3 and SiH4 is
H2S < SiH4 < NH3 < BF3
H2S < NH3 < BF3 < SiH4
NH3 < H2S < SiH4 < BF3
NH3 < H2S < SiH4 < BF3
Which one the following sets of ions represents the collection of isoelectronic species?
K+ , Ca2+, Sc3+, Cl-
Na+ , Mg2+, Al3+, Cl-
K+ , Cl- , Mg2+, Sc3+
K+ , Cl- , Mg2+, Sc3+
Among Al2O3, SiO2, P2O3 and SO2 the correct order of acid strength is
SO2 < P2O3 < SiO2 < Al2O3
Al2O3 < SiO2 < P2O3 < SO2
Al2O3 < SiO2 < SO2 < P2O3
Al2O3 < SiO2 < SO2 < P2O3
The bond order in NO is 2.5 while that in NO+ is 3. Which of the following statements is true for these two species?
Bond length in NO+ is greater than in NO
Bond length is unpredictable
Bond length in NO+ in equal to that in NO
Bond length in NO+ in equal to that in NO
The states of hybridization of boron and oxygen atoms in boric acid (H3BO3) are respectively
sp2 and sp2
sp3 and sp3
sp3 and sp2
sp3 and sp2
